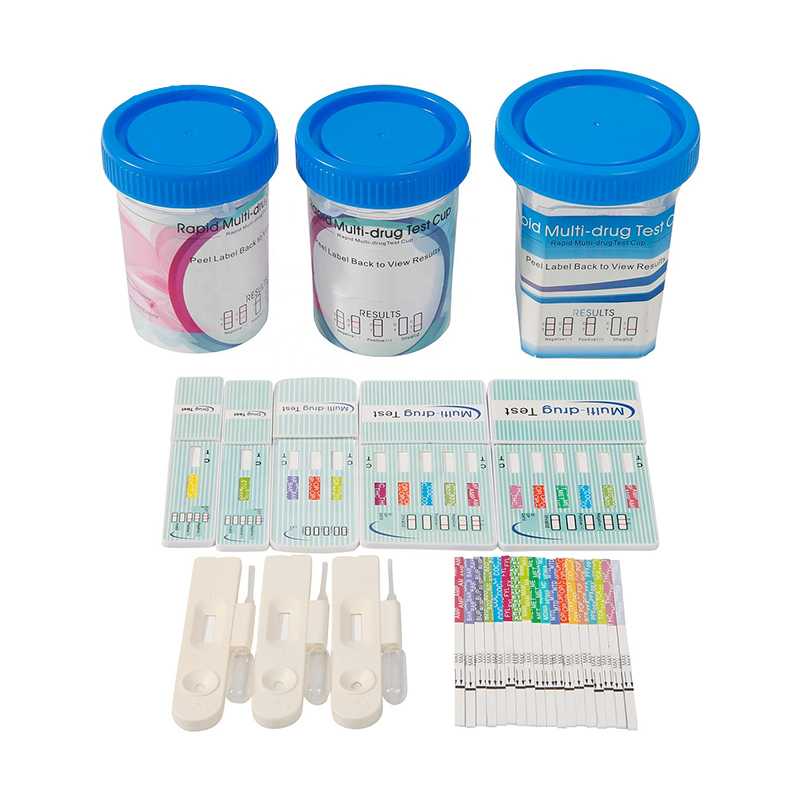


High Accuracy Drug of Abuse Rapid Screening Test Kit
Multi-drug Rapid Test Dipcard is a rapid screening test for the qualitative detection of multiple drugs and drug metabolites in human urine at specified cut-off levels. These tests are simple, fast reliable, and accurate tests that detect the presence of drugs of abuse in urine.
- SKU:
- Category: Human Rapid Test
It can be used for the following drug screening:
Alcohol Ethylglucoronide Test
Acetaminophen (ACE)
Amphetamine (AMP)
Barbiturates (BAR)
Benzodiazepines (BZO)
Buprenorphine (BUP)
Cocaine (COC)
Cotinine (COT)
Ethyl Glucuronide (ETG)
Fentanyl (FYL)
Ketamine (KET)
Marijuana (THC)
Methadone (MTD)
Methamphtamine (MET)
Methaqualone (MQL)
Methylenedioxymethamphetamine-Ecstasy (MDMA)
Opium (OPI)
Opiate 2000
Oxycodone (OXY)
Phencyclidine (PCP)
Propoxyphene (PPX)
Tri-cyclic Antidepressants (TCA) Test
Synthetic K2
6-Monoacetylmorphine (MAM)
Usage:
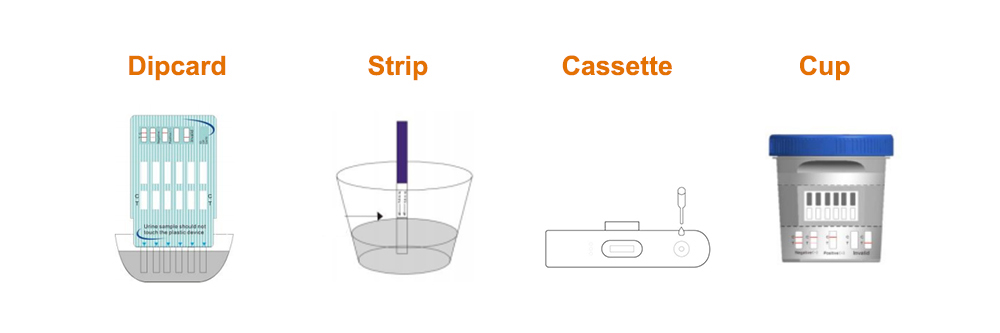
For Strip:
The test must be at room temperature 59-86°F (15-30°C).
•1 Open the sealed pouch by tearing it along the notch. Remove the test strip from the pouch.
•2 Immerse the strip into the urine with the arrow pointing towards the urine. Take the dip card out after 10 seconds.
IMPORTANT: Do not allow the urine level to exceed the MAX (marker line), otherwise the test will not perform correctly.
•3 Lay the test dip card on a clean, dry, non-absorbent surface.
•4 Wait for colored lines to appear. Read the displayed result within 5 minutes, and the results read after 30 minutes is invalid.
For Cassette:
•1 Remove the test cassette from the sealed pouch.
•2 Hold the dropper vertically and transfer 3 full drops (approx. 100ml) of urine to the specimen well of the test cassette, and then begin timing.
•3 Wait for colored lines to appear. Read the displayed result within 5 minutes, and the results read after 30 minutes is invalid.
For Dipcard:
•1 Remove the test card from the sealed pouch and use it as soon as possible.
•2 Remove the cap from the end of the test card. Immerse the strip(s) of the test card vertically in the urine specimen with arrows pointing toward the urine specimen. Immerse the test card to at least the level of the wavy lines on the strip(s), but not above the arrow(s) on the test card.
•3 Remove the test card after 10 seconds and lay the card flat on a non-absorbent surface, and then begin timing.
•4 Wait for colored lines to appear. Read the displayed result within 5 minutes, and the results read after 30 minutes is invalid.
For Multi-Drug Screen Test Cup:
•1 Remove the test cup from the sealed pouch and use it as soon as possible.
•2 Donor provides specimen and secures the cap by pressing down on all three corners.
•3 On a flat surface, technician pushes key to a fully closed position.
•4 Peel off the label on the multi-drug test card to view results. The test is read in the reaction well.
•5 Start the timer and wait for colored lines to appear. Read the displayed result within 5 minutes, and the results read after 30 minutes is invalid.
Interpretation of results:
Positive: One red line appears in the control region (C). No line appears in the test region (T). This positive result indicates that the drug concentration is above the detectable level.
Negative: Two lines appear. One red line should be in the control region (C), and another apparent red or pink line adjacent should be in the test region (T). This negative result indicates that the drug concentration is below the detectable level. (*Note: The shade of red in the test line region (T) will vary, but it should be considered negative whenever there is even a faint pink line.)
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test panel. If the problem persists, discontinue using the lot immediately and contact us.
Warnings and precautions:
• Avoid cross contamination of urine samples by a new sample collection container and pipette for each urine sample.
• Urine specimens may be potentially infectious. Avoid contact with skin by wearing gloves and proper laboratory attire. Properly handle and dispose of all used test devices in an approved biohazard container.
• Do not use a test device beyond the expiration date imprinted on the outside of the foil pouch.
Storage:
Store at 2 ~ 30 º C in the sealed pouch for 24 months.
The test must remain in the sealed pouch until use.
Keep away from direct sunlight, moisture and heat.
Do not freeze.
Additional Information
Product Type: In Vitro Diagnostic Equipment
Formats: Strip, Cassette, Dipcard, Cup
Specimen: Urine, Saliva, Blood
Response time: 5 mins
Accuracy: ≥99.96%
OEM/ODM: Available
Place of origin: China
Packaging Details:
•1 Standard Export Package:Sterile Package + Standard Export Carton
•2 OEM Package: Customized inner bag + Customized inner box + Customized export carton
Send an Inquiry
Your email address will not published. Required fieled are marked.
Related Products
Check out other related DNA/RNA Extraction Products
