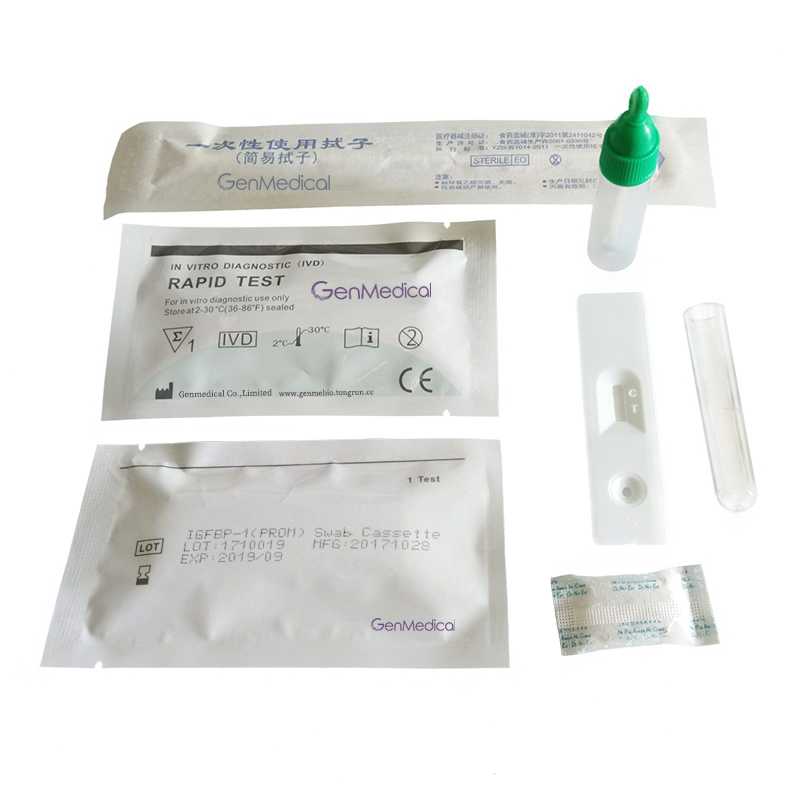

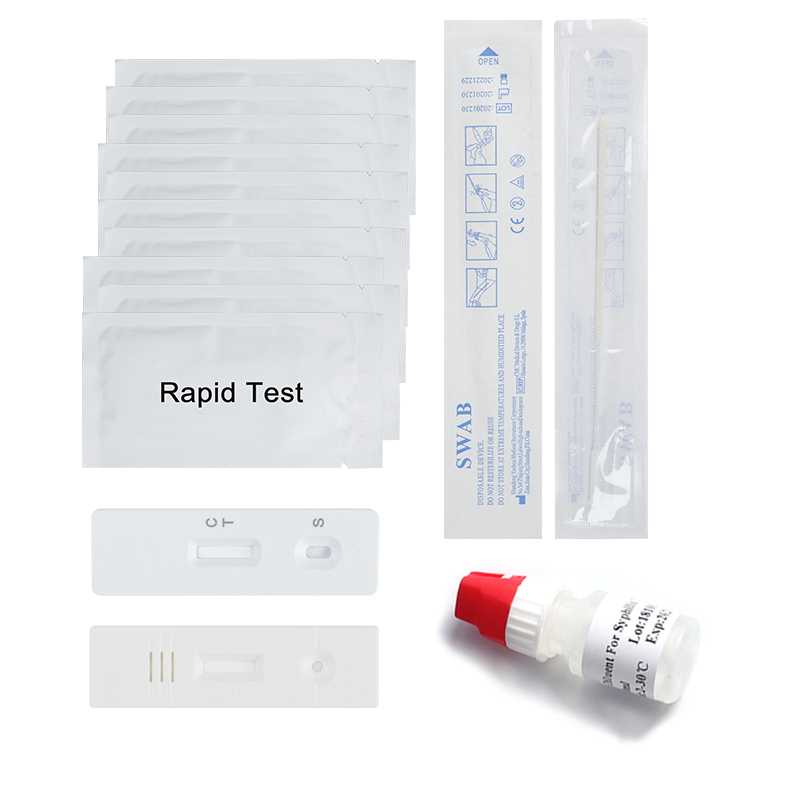
Human Amniotic Fluid IGFBP-1 Swab Rapid Test Kit
Human Amniotic Fluid IGFBP-1 Swab Rapid Test Kit is intended for professional use to help diagnose the rupture of fetal membranes (ROM) in pregnant women.
- SKU:
- Category: Human Rapid Test
The Insulin-like Growth Factor Binding Protein IGFBP-1 Rapid Test Strip (Cervical Secretion) is a visually interpreted, qualitative immunochromatographic dipstick test for detection of IGFBP-1 in cervical secretions during pregnancy, which is a major protein marker of the amniotic fluid in a cervical sample.
Usage:
•1 Remove the tests from its sealed pouch, and place it on a clean, level surface. Label the test with patient or control identification. To obtain a best result, the assay should be performed with in one hour.
•2 Insert the swab into the dilute tube, rotate for about 20 times. Then press the swab against the side of the tube and squeeze the bottom of the tube as the swab is withdrawn. Discard the swab.
•3 Fit the cap of the tube. Remove the top part of the cap. Place the test device on a clean and level surface. Add 3 full drops of solution(approx. 100µl) to the specimen well(S) and then start the timer.
•4 Wait for the colored band to appear. The result should be read at 5~10minutes.
Interpretation of results:
•1 A colored band appears in the control band region(C) and another colored band appears in the T band region.
•2 One colored band appears in the control band region(C). No band appears in the test band region(T).
•3 Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, please contact customer service.
Note:
1.The intensity of the color in test region(T) may vary depending on the concentration of aimed substances present in the specimen. Therefore, any shade of color in the test region should be considered positive. Besides, the substances level can not be determined by this qualitative test.
2. Insufficient specimen volume, incorrect operation procedure, or performing expired tests are the most likely reasons for control band failure.
Feature:
• Easy use without extra laboratory equipment
• Two band results for simple interpretation
• Rapid results reading within 5 minutes
Additional Information
Product Type: Pathological Analysis Equipment
Application: Women Pregnancy Predicts Amniotic Fluid Early
Formats:Cassette
Material: Plastic
Kit size: 20tests/Kit
Sensitivity: 25ng/ml
Response time:within 5mins
Specimen: Vaginal Secretions
Accuracy: ≥ 99%
Storage: Room temperature storage or refrigerated (2-30ºC)
Shelf time: 24 months
OEM/ODM: Available
Place of origin: China
Packaging Details:
•1 Standard Export Package:Sterile Package + Standard Export Carton
•2 OEM Package:Customized inner bag + Customized inner box + Customized export carton
Send an Inquiry
Your email address will not published. Required fieled are marked.
Related Products
Check out other related DNA/RNA Extraction Products
